High vapour pressure goes to low vapour pressure
High vapour pressure goes to low vapour pressure
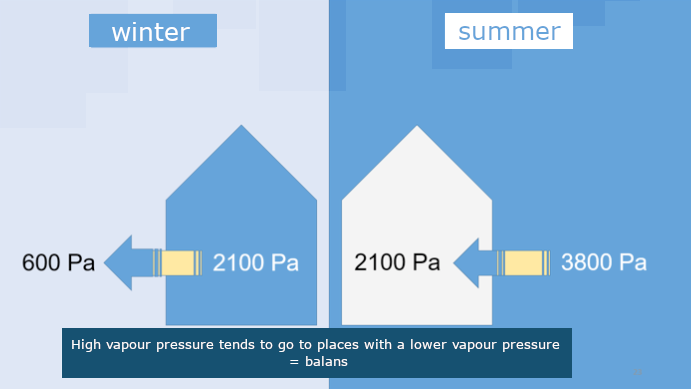
A higher vapour pressure always goes to places where the vapour pressure is lower. When you blow up a balloon and let is go untied, it flies through the room. This is kind of the same.
Almost always the vapour pressure in a house, building or apartment is higher than outside wherefor there’s always moisture transport from inside to outside. Almost always that is. On hot summer days it can occur than the vapour pressure outside is much higher than inside. At +21°C and 80% rH there is outside a vapour pressure of 3800 Pa. The higher vapour pressure from outside is then pushed inside and is absorbed. This is only a few days a year. And with such hot weather the windows and doors are usually open.
In a house the average temperature is +21°C with a relative humidity of about 50%. Then there is a vapour pressure of 1244 Pa. If it cools down at night to 0°C with misty weather and 98% rH, then there’s outside only a vapour pressure of 600 Pa. That’s less than half of the normal vapour pressure inside.
Since everything wants to stay in balance, so the higher vapour pressure inside will be pushed through the walls to places with much lower vapour pressure, so outside.
The problem isn’t that the vapour pressure and the absorbed moisture goes through the wall to outside, but that it get over the dew point in the cavity wall and the moisture condensates. Because of this moisture damage occurs and possible freeze damage in long term. Even if the used construction materials are resistant to absorption and discharge of moisture, then it still costs a lot of energy construction physically seen. How can you live energy neutral tomorrow if the absorption and discharging of moisture costs 30% of the heating cost?


